Our Services
Lack of clinical safety is a major cause of failure of drug development programmes, and adverse effects on vital organs are often responsible. Vivonics can help your project team identify potentially undesirable effects of drugs on vital organ function using validated preclinical in vivo assays.
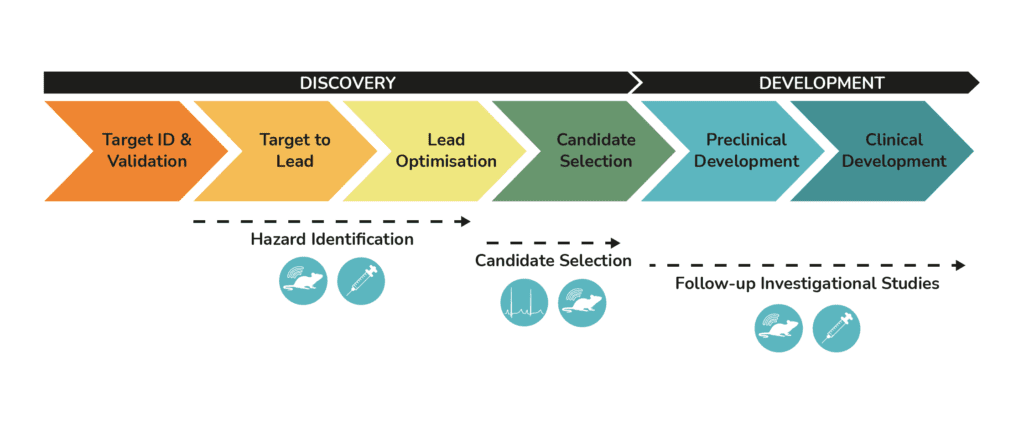
We have expertise in assessing cardiovascular safety utilising small animal conscious telemetry models and studies under terminal anaesthesia. These assays can be used for hazard identification during the drug discovery phase. Alternatively, they may be used as follow-up studies to better characterise adverse effects, to identify the mechanism of action, or to investigate pathological findings in prior toxicology studies. Our assays can also be used to assess the effects of your lead molecules on the central nervous system and the respiratory system.
The data we generate provides your team with an early assessment of safety, helping you optimise molecules, assess risk/benefit and identify drug candidates with better safety profiles for further development.

Telemetry
Investigating effects on physiological parameters in conscious, freely moving animals
In Vivo QT Screen
An early in vivo assessment of the effects on cardiac electrophysiology
Anaesthetised Models
For screening, investigating mechanism of action or problem solving studies
Blood & Tissue Samples
For test compound analysis, biochemical and histopathological endpoints

