Anaesthetised Models
Anaesthetised studies in rats, and also mice, have been shown to be useful when utilised in the early phase of drug discovery to assess on- or off-target safety liabilities and also in later phase problem solving or investigatory studies*. There is a good concordance of drug-induced cardiovascular effects between this model and larger conscious animals and humans. Anaesthesia can blunt baroreceptor reflexes, but this may make the model more sensitive to detecting underlying cardiovascular changes and may also emulate the situation in many patient populations with reduced baroreceptor sensitivity (e.g. the elderly, hypertensive patients). We have experience of using many different anaesthetics and can advise on the most appropriate agent to use depending on the purpose of the study.
Test drugs are usually administered intravenously which means only small amounts of test drug are required. Blood samples for concentration analysis can be obtained without any stress-induced cardiovascular artefact and it is usually possible to explore a larger dose range than in conscious animals due to the absence of dose-limiting behavioural effects.
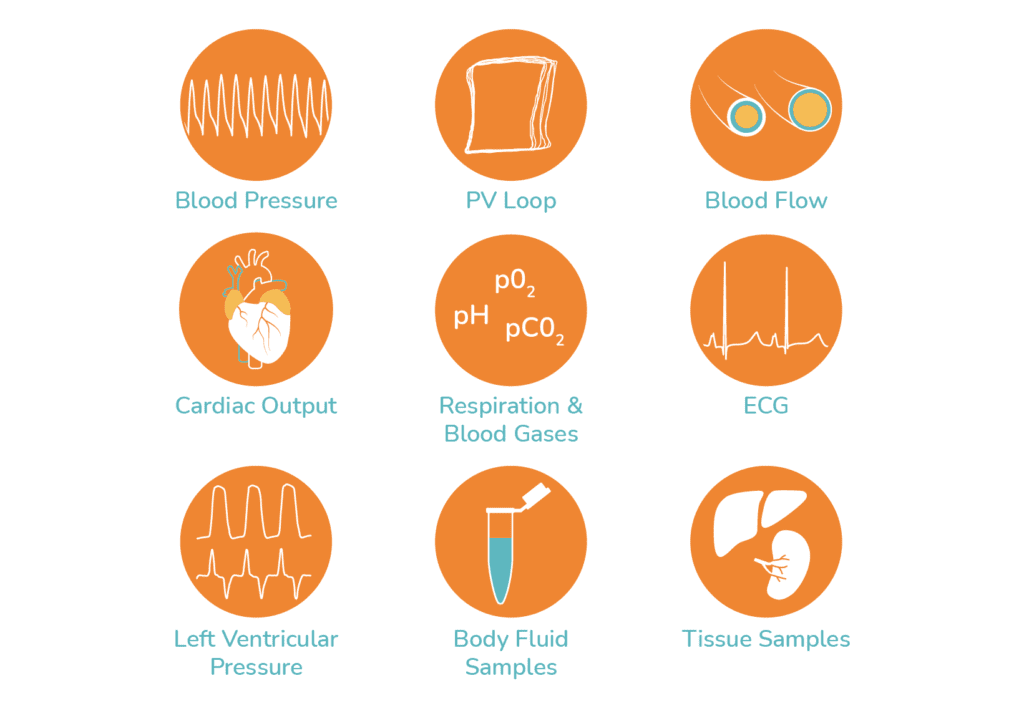
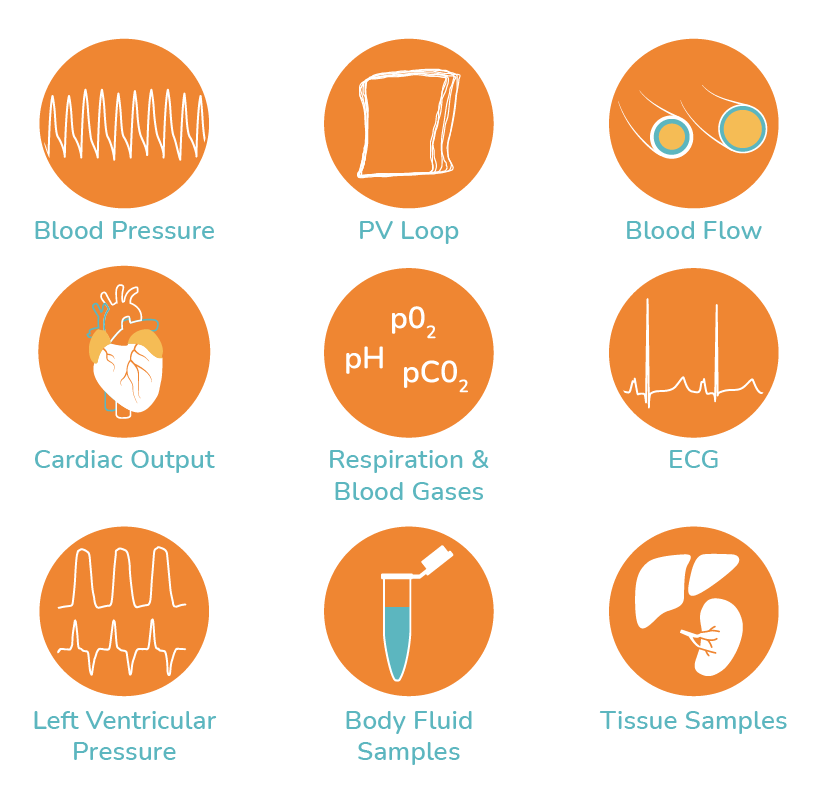
The model allows a greater variety of physiological endpoints to be measured making it amenable for more detailed studies investigating mechanisms of acute cardiovascular toxicity. Parameters able to be measured include: arterial and venous blood pressures, left and right ventricular pressures, pressure-volume (P-V) loops , cardiac output and stroke volume, ECG, blood flow in various vascular beds, respiratory parameters, blood gas, chemistry and metabolism. Urine and tissue samples may also be taken.
*Ref Skinner et al., The contribution of VEGF signalling to fostamatinib-induced blood pressure elevation. Br J Pharmacol. 2014 May;171(9):2308-20.